40 fda guidance use of symbols on labels
› obp › uiISO 15223-1:2021(en), Medical devices ? Symbols to be used ... IEC 80416-3: 2002+A1:2011, Basic principles for graphical symbols for use on equipment — Part 3: Guidelines for the application of graphical symbols [21] EN 556-1:2001, Sterilization of medical devices — Requirements for medical devices to be designated “STERILE” — Part 1: Requirements for terminally sterilized medical devices en.wikipedia.org › wiki › LabelLabel - Wikipedia These labels include information like brand name, class and type designation, and alcohol content. Packaging. Packaging may have labeling attached to or integral with the package. These may carry pricing, barcodes, UPC identification, usage guidance, addresses, advertising, recipes, and so on. They also may be used to help resist or indicate ...
Publication of 'healthy' definition and draft guidance on plant-based ... The FDA - which had previously announced its intention to release draft guidance on plant-based milk labeling by the end of June, told us: "The FDA intends to release a draft guidance expected ...

Fda guidance use of symbols on labels
FDA Issues Final Rule on Use of Symbols in Labeling - Lexology the final rule allows symbols established in a standard developed by a standards development organization to be used in medical device labeling without adjacent explanatory text if (1) the standard... Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 801.1 - Medical devices; name and place of business of manufacturer, packer or distributor. § 801.3 - Definitions. § 801.4 - Meaning of intended uses. § 801.5 - Medical devices; adequate directions for use. Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer ... The FDA authorized emergency use of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include use in children down to 6 months of age.
Fda guidance use of symbols on labels. Federal Register :: Use of Symbols in Labeling accordingly, this final rule provides that a stand-alone symbol is allowed to be used in device labeling if: (1) the symbol is established in a standard developed by an sdo; and (2) the standard is recognized by fda under its authority under section 514 (c) of the fd&c act and the symbol is used according to the specifications for use of the … New Website for Med Device Labeling Symbols - Healthcare Packaging The FDA has launched a new website to help the public understand the Use of Symbols in Labeling Final Rule. In June of 2016, the FDA issued a final rule, Use of Symbols in Labeling, which allows for the use of symbols in all medical device labeling without additional explanatory text. 21 CFR § 801.15 - Medical devices; prominence of required label ... (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ... Summary: Use of Certain Symbols in Labeling | FDA Summary: Use of Certain Symbols in Labeling The proposed rule would provide medical device manufacturers with the option to use standardized international symbols (recognized by the FDA) to...
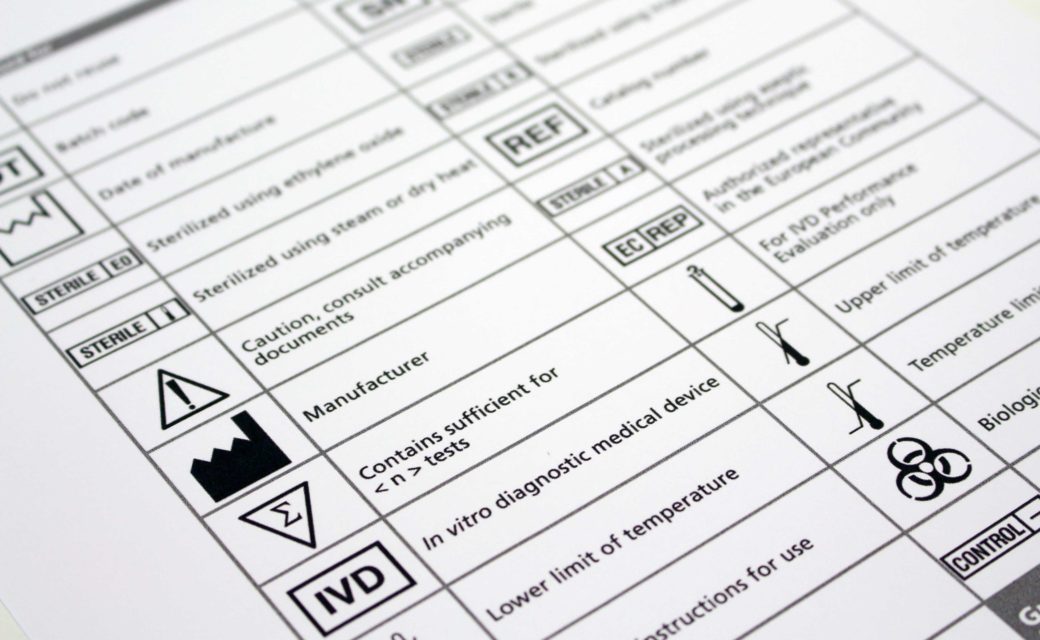
Use of Symbols on Labels and in Labeling of In Vitro ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553. PDF Symbols Commonly Used in Medical Device and Packaging Labeling Caution: Federal law restricts this device to sale by or on the order Use of the "Rx only" symbol as an alternative to the prescription device labeling statement. FDA Guidance Alternative to Certain Prescription Device Labeling Requirements N/A 2. Alarm to indicate an alarm on a control equipment IEC 60601‐1‐8 Table C1, No. 1 3. › medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA The Use of Symbols in Medical Device Labeling Final Rule ("final rule"), including the requirement for a glossary, only applies to symbols that are used to convey information required by or under... bloodbook.com › FDA-inspectFDA Guide to Inspections of Blood Banks - BloodBook, Blood ... For additional guidance, see the July 29, 1994 memorandum, "Use of an FDA Cleared or Approved Sterile Connecting Device (STCD) in Blood Bank Practice." BLOOD COLLECTION CONTAINER Names of blood container manufacturers may be found on the labels of the blood bags.
› about-fda › economic-impact-analysesSummary: Use of Symbols in Labeling (Final Rule) | FDA The final rule also specifies that the use of symbols, accompanied by adjacent explanatory text continues to be permitted. FDA is also revising its prescription device labeling regulations to allow... FDA Issues Final Rule on Symbols for Device Labels | RAPS In the past, FDA prohibited companies from using standalone symbols on device and IVD labels, and required symbols on the labels to be accompanied by explanatory text. However, in 2013, after pressure from the medical device industry, FDA issued a proposed rule that would allow standalone symbols to appear on device labels for public comment. › business-guidance › resourcesAdvertising FAQ's: A Guide for Small Business | Federal Trade ... Apr 04, 2001 · The FDA handles most matters regarding food labels. For guidance on how the FTC evaluates claims made in food ads, ask the FTC for the Enforcement Policy Statement on Food Advertising. For information about product labeling, visit the FDA's website at or call the FDA Inquiry Line, 1-888-INFO-FDA Use of Symbols in Labeling | FDA Use of Symbols in Labeling The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits...
FDA Issues Final Rule Permitting Use of Symbols on Device Labeling Three years ago, FDA proposed a rule that would permit device manufacturers to use stand‑alone symbols on device labeling. In the meantime, this issue has continued to fester. So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways:
The FDA's Guidance on Labels and Cartons: yet another ... - pharmaphorum Most importantly, the FDA intends to actively discourage the use of logos, bars, stripes, watermark graphics, lines, and symbols on container labels and / or carton labeling because they can ...
Using Symbols to Convey Information in Medical Device Labeling | FDA The use of stand-alone symbols on a global scale may help promote better understanding through consistent labeling across products distributed in the U.S. and foreign markets. Before this rule, FDA...
Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by FDA's labeling requirements for IVDs. This draft guidance is not final nor is it in effect at this time. DATES:
eCFR :: 21 CFR Part 801 -- Labeling (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ...
Device Labeling | FDA Labeling regulations pertaining to medical devices are found in the following Parts of Title 21 of the Code of Federal Regulations (CFR). General Device Labeling - 21 CFR Part 801 Use of Symbols -...
Code of Federal Regulations Title 21 - Food and Drug Administration (c) (1) (i) All words, statements, and other information required by or under authority of the act to appear on the label or labeling for a device shall appear thereon in one or more of the...
Final rule on use of symbols on labeling - Symbols without text under ... Jun 14, 2016. #4. Jun 14, 2016. #4. FDA issues final rule on use of symbols in labeling. Today the Food and Drug Administration (FDA) issued a final rule "Use of Symbols in Labeling" allowing the use of stand-alone symbols in medical device labeling, without adjacent explanatory text.
Use of Symbols in Labeling - US Food and Drug Administration FDA assumes that the labeling of each uniquely labeled medical device can communicate the same information using -alone standsymbols or symbols with adjacent explanatory text. FDA further assumes...
PDF Use of Symbols on Medical Device Labels - FDA Requirements In all such cases, we use EN 980/ISO 15223 symbology on the label, with English-only subtext for each symbol in a small but readable font size. We have been doing this for a number of years now, and have had no objection from US FDA, our NB, our Authorized Representative, authorities in Europe, or end users. adv_webdev24th June 2011 04:45 PM
› usda-food-label-gmoGMO Foods Will Be Labeled 'Bioengineered' - Verywell Health Jan 07, 2022 · Studies have shown that genetically engineered foods are just as safe to consume as foods that have not been genetically engineered. If you decide to avoid genetically engineered foods, keep an eye out for these new labels on grocery store shelves. But remember, certain foods and producers are exempt from using the new bioengineered labels.
Recognized Consensus Standards - Food and Drug Administration Recognized Consensus Standards. This document specifies symbols used to express information supplied for a medical device. This document is applicable to symbols used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements. These symbols can be used on the medical device itself, on ...
Use of Symbols in Medical Device Labeling- FDA's Final Rule The rule finalizes the FDA's 2013 proposed rule and affects biologics, IVDs, and medical devices regulated by 21 CFR Parts 660, 801, and 809. The revision allows for the inclusion of symbols in labeling without adjacent explanatory text (referred to as "stand-alone symbols"), so long as certain requirements are met.
Labeling and Label Approval | Food Safety and Inspection Service Labeling and Label Approval. FSIS develops and provides labeling guidance, policies and inspection methods and administers programs to protect consumers from misbranded and economically adulterated meat, poultry, and egg products which ensure that all labels are truthful and not misleading. FSIS revised guidance on food product date labeling to ...









Post a Comment for "40 fda guidance use of symbols on labels"